Pharmacovigilance: Monitoring and Reporting Adverse Drug Reactions
Keywords:
Adverse Drug Reactions, Active Surveillance, Spontaneous Reporting, Meddra Coding.Abstract
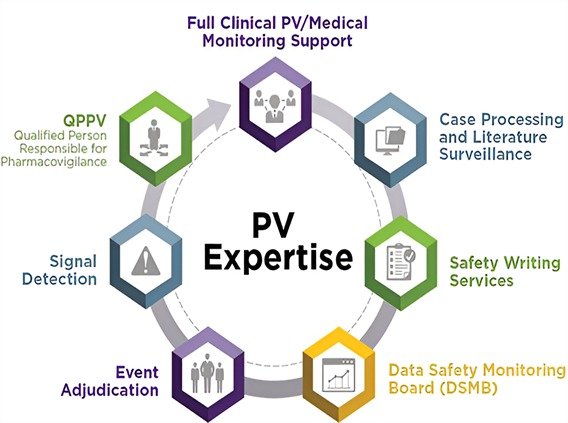
Pharmacovigilance plays a pivotal role in ensuring the safety and efficacy of pharmaceutical products throughout their lifecycle. Monitoring and reporting adverse drug reactions (ADRs) are fundamental components of pharmacovigilance, aimed at identifying and minimizing risks associated with medication use. This paper provides an overview of the pharmacovigilance process, emphasizing the importance of robust ADR monitoring and reporting systems. It explores the various methods employed in pharmacovigilance, including spontaneous reporting, literature review, observational studies, and signal detection techniques. Additionally, the paper discusses the challenges and opportunities in ADR monitoring, such as underreporting, signal refinement, and the emergence of new data sources like social media and electronic health records. Strategies to enhance ADR reporting, including education and training initiatives for healthcare professionals and patients, as well as the utilization of advanced technology and data analytics, are also examined. Furthermore, the role of regulatory agencies, pharmaceutical companies, healthcare providers, and consumers in promoting pharmacovigilance efforts is highlighted.
Published
How to Cite
Issue
Section
Copyright (c) 2022 Authors

This work is licensed under a Creative Commons Attribution 4.0 International License.




