The pH of Drinking Water and Its Human Health Implications: A Case of Surrounding Communities in the Dormaa Central Municipality of Ghana
Keywords:
Acidic Environment, Potential For Hydrogen (Ph), Drinking Water, Heavy Metal Poisoning, Health Effects.Abstract
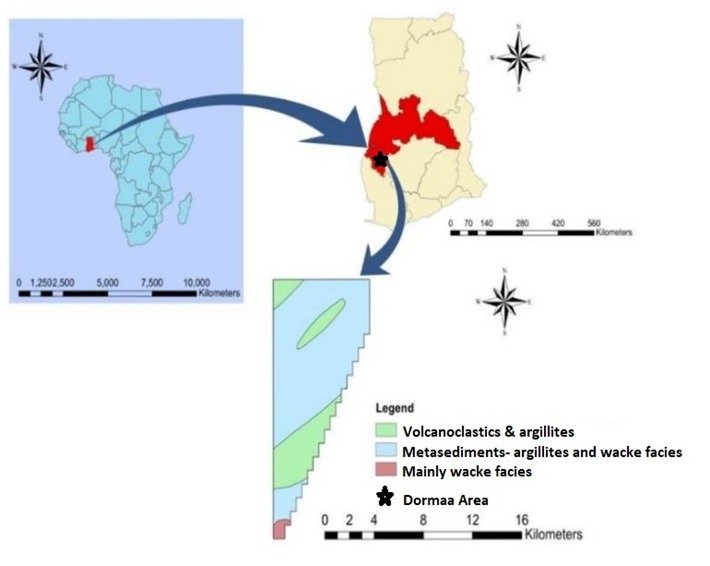
Of all the things to consider about safe water, the pH of drinking water probably has been overlooked. Most spoken about, however, is alkaline water which has a host of supposed health benefits? For instance, it is known to help the body to clear toxins thereby improving metabolism. Meanwhile, research has shown that most diseases, illnesses, and bad bacteria thrive in an over-acidic environment. Additionally, the acidic water indirectly may impact on budget as this would contribute to the metallic or sour taste of drinking water, and stained laundry and provide blue-green staining of sinks and other household fixtures. Acidic water having low pH often are known to contain high amounts of heavy metals. Also, research has found that solutions with low pH are more likely to have heavy metals from the environment. Other researchers have identified that acidic water can be high in Pb, As, Cu, Ni, Cb, Cr, and Zn. All these elements fall under heavy metals and exposure to them can be dangerous, and could lead to heavy metal poisoning and toxicity. This is concerning as water is said to be life and the population within Dormaa Central Municipality is most likely to have symptoms such as diarrhoea, nausea and vomiting, abnormal pains, weakness, shortness of breath, suppression of the immune system, organ damage, and enamel wearout leading to dental cavities. Water samples and their corresponding spatial locations were collected from (how many?) communities within the Dormaa Central Municipality. The potential for hydrogen (pH) readings of the respective water samples was measured using a pH meter. The results obtained range from 0.2 mmHg to 6.5 mmHg.
Published
How to Cite
Issue
Section
Copyright (c) 2023 Emmanuel Arhin, Jeff Dacosta Osei, Prisca Ama Anima, Peter Damoah-Afari, Lily Lisa Yevugah

This work is licensed under a Creative Commons Attribution 4.0 International License.




